So you’ve waded through the complexity of Part 1, gaining a foundational understanding of soil building. In this second installment, we’re diving even deeper into the science of soil. Specifically, we’ll look at how the Cation Exchange Capacity (CEC) serves as the foundation for deciding nutrient amendments, and why balancing your soil is like concocting a fine, artisanal brew—unique, complex, and absolutely vital for top-tier cannabis growth.
The Importance of CEC in Soil Building
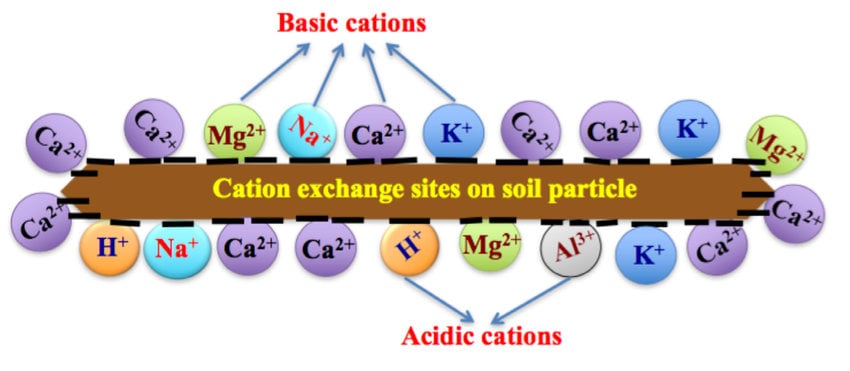
First and foremost, let’s revisit CEC. Standing for Cation Exchange Capacity, CEC is essentially the soil’s ability to hold onto essential cations—positively charged nutrient ions. CEC is affected by several factors:
- Clay Content: Soils rich in clay have CEC. This is because clay particles are negatively charged, allowing them to latch onto positively charged cations. While the exact ideal percentage of clay hasn’t been nailed down, know that too much can cause aeration issues.
- Organic Matter: Organic matter also increases CEC due to its negative charge. Cornell University’s research suggests that an ideal soil contains around 20% organic matter.
- Inert Substrates: These include options like peat moss, which has CEC, and coco coir, with a more moderate CEC. Peat moss is packed with humates that add to its negative charge, while coco coir offers excellent aeration, despite having lower CEC compared to peat moss.
Detailing Your Nutrient Amendments Based on CEC and Target Values
Understanding your soil’s Cation Exchange Capacity (CEC) is crucial for determining the precise amounts of various elements needed for optimal plant growth. This becomes particularly important when you’re growing specific crops, such as cannabis. Below, we provide a detailed list of essential nutrients, their optimal amounts in parts per million (ppm) or as a percentage of CEC, and their atomic weights in grams per mole (g/mol).
Essential Nutrients and Their Ideal Values
| Element | Ideal Value | Atomic Weight (g/mol) |
|---|---|---|
| Nitrogen | 150-250 ppm | 14.01 |
| Phosphorus | Equal to potassium value | 30.97 |
| Potassium | 2-5% of CEC | 39.10 |
| Calcium | 60-70% of CEC | 40.08 |
| Magnesium | 10-20% of CEC | 24.31 |
| Sulfur | ⅓ of potassium | 32.07 |
| Silicon | 50-30 ppm | 28.09 |
| Iron | 2-4 ppm | 55.85 |
| Manganese | 1-2 ppm | 54.94 |
| Zinc | 1/10 of phosphorus | 65.38 |
| Copper | ½ of zinc | 63.55 |
| Boron | 0.5-1 ppm | 10.81 |
| Molybdenum | 0.01-0.05 ppm | 95.95 |
Remember, your soil is like a chain: it’s only as strong as its weakest link. Ensuring that each of these components is present and in the right proportions will help you create an optimal growing environment.
Step-by-Step Nutrient Calculation: An Example with Calcium
Understanding the theory is great, but applying it practically is where you can truly optimize your soil. In this section, we’ll walk through a detailed example to calculate the amount of calcium needed for soil with a CEC of 20. Note that this calculation assumes the soil is completely void of calcium.
The Calculation Explained
- Identify Desired Percentage: Decide the percentage of the cation exchange sites you want to be occupied by the nutrient. For calcium, an ideal percentage is 70% (or 0.7 in decimal form).
- Calculate Milliequivalent (meq): Multiply the CEC value by the desired percentage in decimal form. For example, 20 (CEC) x 0.7 = 14 meq.
- Convert meq to Moles: Use the formula:
Moles = meq/100g * (1mol/1000 meq) * (2/ Atomic Weight of Cation). In our example, this becomes 14 meq/100g * (1mol/1000meq) * (2/40.08) = 0.000699 mol. - Convert Moles to Grams: Multiply the mole value by the atomic weight of the cation (calcium in this case is 40.08 g/mol). For example, 0.000699 mol * 40.08 g/mol = 0.028 grams.
- Convert Grams to ppm: Convert the grams to parts per million (ppm) by multiplying by 1,000,000. For instance, 0.028 grams becomes 28,000 ppm.
So, if your soil has a CEC of 20 and you aim for 70% of the cation exchange sites to be occupied by calcium, you would need 28,000 ppm of calcium. Remember that this is a theoretical example and the actual values may vary based on several factors including existing nutrient levels and soil volume.
Calculating Deficits: A Detailed Example Using Gypsum
Let’s dive into a real-world example to understand how you would calculate the amount of gypsum (calcium sulfate) needed to correct deficits in calcium and sulfur in your soil.
Step-by-Step Calculation
- Determine Soil Volume: For this example, let’s assume you’re working with one cubic yard of soil.
- Conduct Laboratory Testing: Obtain lab tests to identify the existing nutrient levels of calcium and sulfur in your soil.
- Identify Nutrient Deficit: Subtract the existing nutrient levels from your target levels. For instance, if your soil needs 200 ppm of calcium and 10 ppm of sulfur, these are your deficit values.
- Check Gypsum Composition: Gypsum typically contains 23% calcium and 18% sulfur. These values may vary based on the quality of your gypsum.
- Calculate Amount of Gypsum Needed: Divide the deficit for each nutrient by the respective percentage of the nutrient in gypsum. For example, a 200 ppm calcium deficit divided by 0.23 (23% calcium in gypsum) equals 869.6 ppm of gypsum. A 10 ppm sulfur deficit divided by 0.18 (18% sulfur in gypsum) equals 55.6 ppm of gypsum.
- Start with the Higher Value: In this case, the calcium value is higher (869.6 ppm), so we’ll start there. However, take note of the sulfur value (55.6 ppm) as well, for a balanced nutrient profile.
By following this process, you can accurately determine how much gypsum you need to bring the nutrient levels within the ideal range. Keep in mind that these calculations are examples and the actual requirements may vary based on several factors.
Crunching Numbers with Parts Per Million (PPM)
When dealing with soil amendments, understanding the concept of Parts Per Million (PPM) is critical. This unit measures nutrient levels in soil. Let’s say you have a calcium deficit of 200 PPM. In a million pounds of soil, this translates to a requirement of 200 lbs of calcium.
Now, consider your soil volume. A cubic yard of soil weighs approximately 2,000 lbs. To find out how much calcium is needed, we perform a quick calculation:
Since gypsum is 23% calcium, we divide 0.4 lbs by 0.23 to find that we need 1.74 lbs of gypsum to resolve a 200 PPM calcium deficit in one cubic yard of soil.
Creating a Balanced Soil Mix: Ratio Matters
When using multi-nutrient amendments like langbeinite and crab meal, you’ll need to balance each nutrient between sources. If both provide potassium, their combined contribution should meet but not exceed your potassium target.
Step-by-Step Soil Creation
- Choose your base substrate, e.g., coco coir or peat.
- Add aeration elements, such as pumice or lava rock.
- Mix in organic matter, like compost or earthworm castings.
- Send a soil sample for testing to determine existing nutrient levels.
- Calculate the needed amendments based on the test results and add them in the order of calcium, phosphorus, and potassium.
- Re-test the soil to verify the nutrient balance before adding trace minerals like copper, zinc, iron, etc.
Final Thoughts: The Art and Science of Soil Building
Building soil for cannabis cultivation is both an art and a science. It’s intricate and often complex, requiring deep mathematical understanding and strategic planning. This complexity translates into a unique soil recipe that brings out unique characteristics in your cannabis. Therefore, keep your soil recipe close to your chest—it’s the secret sauce of your cultivation prowess.
Remember, while creating the perfect soil mix can be laborious, the rewards are bountiful: healthy plants with unique flavor profiles and qualities. Soil building isn’t just about growing plants; it’s about growing the best version of your plants. Happy cultivating!
About the Author:
Luna Whitcomb is a seasoned cannabis cultivator with over 14 years of experience across various cultivation methods, from living soils to hydroponics. Specializing in organic cannabis cultivation, Luna has mastered the art of creating nutrient-rich, living soils that promote robust plant health and yield. As an educational content creator, Luna shares her wealth of knowledge on platforms like SKUNK Magazine, Patreon, and YouTube, offering consultations and even writing cannabis license applications for large-scale operations. Her comprehensive approach to cultivation combines elements of microbiology, botany, agronomy, and more, all aimed at producing cannabis of superior quality and rich compound profiles. Currently based in Oregon, Luna continues to push the boundaries of cannabis cultivation, influencing both growers and consumers towards eco-conscious and scientifically-backed practices.

Very informative read, but I didn’t get a chance to read part one before it was taken off the post., all in all green read. Thanks to luna whitcomb and green point I learned a lot.